- Guaranteed Secure Payments on Every Order
- Refund if your item is not delivered or as described
- Buyer Protection after order confirmation
- Size: 0.049
- Place of Origin: Heze City ,Shandong Province
- Brand Name: Huangying
- Model Number: 500ml
- Weight: 23.000 kg
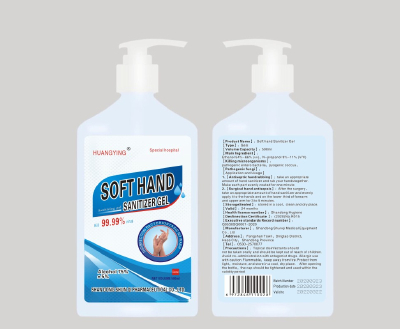
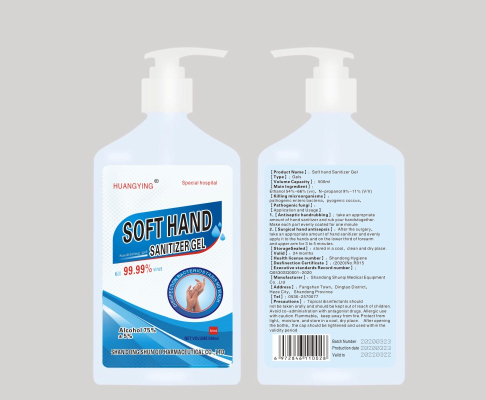
Soft hand Sanitizer Gel
1 . Scope
This standard states the requirements of no-wash hands sanitizer gels (hereinafter referred to as: sanitizer gels) for its raw materials, test methods, inspection rules, marking, packaging, shipping, storage, labeling, and instructions.
This standard applies only to no-wash hands sanitizer gels.
2. Reference standards
The following standards includes provisions that, through application in this standard, constitute provisions of this standard.
At the time of publication of this standard, the editions shown are valid and all standards can be revised. The methods of using this standard should explore the possibility of using the latest versions of the following standards.
Disinfection Technical Norm Year of 2002
GB10434 edible alcohol
GB26373-2010 Hygienic standard for ethanol disinfectants
GB15979-2002 Hygienic Standard for Disposable Sanitary Products
GB191-2008 Standard for Packaging, Storage and Transportation
"Pharmacopoeia of the People's Republic of China" (2015 edition two)
Ministry of Health of the People's Republic of China, 2005 Management Specification for Disinfection Product Labeling Instructions
3 . Composition and specifications
This product is a gel disinfectant based on ethanol and n-propanol; the volume capacity are 30ml / bottle, 50ml / bottle, 80ml / bottle, 100ml bottle, 150ml / bottle, 200m / bottle, 250ml bottle, 300m / bottle, 350ml bottle, 400ml / bottle, 500ml / bottle, 1L / bottle, 1.5 / bottle, 2L / bottle special
The volume capacity can be customized according to customer requirements.
4 . Raw material requirements
The water used in the formula should be pure water; the raw materials in the formula should meet the relevant regulations under the names of the Pharmacopoeia of the People's Republic of China (2015 Edition) or meet the requirements for medical use.
requirements.
5 . technical requirements
5.1 sensory attributes, size
Sensory traits, Size
Sanitizer gels should be colorless and transparent gel, free of impurities, and with the inherent odor of ethanol
5.1.2 Volume
The volume of sanitizer gels should be no less than 95%
5.2 Stability
The packaged Sanitizer gels is valid for two years under the storage conditions specified for the product
5.3 Physical and chemical indicators
5.3.1 PH should be 4.5-8.0
5.3.2 Ingredient:
Ethanol 54%~66% (vv), N-propanol 9%~11% (V/V)
5.4 Microbial index
The total number of bacterial colonies should be less than or equal to 20cFu / g, and the total number of fungal colonies, coliforms, and pathogenic pyogenic bacteria (Pseudomonas aeruginosa, Staphylococcus aureus, and hemolytic streptococcus) shall not be detected.
5.5 Killing microorganism index
Sanitizer gels should has a killing rate of 90% or more on Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Monilia albican
Test method
6.1 Sensory traits, Size
6.1.1Sensory traits
Observe with normal or corrected vision
6.1.2Measurement
Measured with universal gage
6.2 Stability
According to the relevant determination method of Disinfection Technical Norm
6.3 Physical and chemical indicators
6.3.1 PH value
Take an appropriate amount of this product and test it with a PH acidity meter.
6.3.2 Reference methods for the determination of ethanol and n-propanol content References "Simultaneous determination of methanol, ethanol, n-propanol in serum by gas chromatography" and "Pharmacopoeia of the People's Republic of China" (2015 edition)
6.3.2.1 Chromatographic conditions
Chromatographic column (6%) cyanopropylphenyl- (94%) dimethylpolysiloxane (or similar polarity) is a capillary column with a fixed solution. The column temperature is 100 ℃ and maintained for 10min. The inlet temperature is 200 ° C, the monitor temperature is 250 ° C, the headspace bottle equilibrium temperature is 70 ° C, the equilibration time is 30min, the carrier gas purity is N299.999, the column flow rate is 1nmin, the constant pressure, and the split ratio is 10: 1. Makeup gas is 25ml / min, 1230m / min, and air is 300min.
6.3.2.2 preparation of control solution
The anhydrous ethanol 6m and anhydrous n-propanol 1ml were precisely transferred to 100m measuring bottle, the deionized water was dissolved and the volume was fixed, the solution lm1 was precisely transferred to another -100ml measuring bottle, the solution was dissolved with water and the volume was fixed, the solution 5ml was precisely transferred to the headspace bottle, sealed, as the control solution
6.3.2.3 Control solution preparation
Precisely takes a 10ml sample and place it in a 100ml volumetric flask, adds water to dissolve and adjust the volume. Precisely transfer 1ml of the solution into a -100m volumetric flask, add water to dissolve and adjust the volume, and accurately transfer 5ml of the solution into a headspace bottle. Sealed as as comparison.
6.3.2.4 Determination of content
Take the reference solution solution for empty injection, and the peaks will appear in the order of ethanol and n-propanol, and the resolution of each component should meet the requirements. Then take the test sample and the control solution for headspace injection, record the chromatogram, and calculate the content of ethanol and n-propanol in the sample by the peak area according to the external standard method.
6.4 Microbiological indicators
According to the method specified in GB15979200 Appendix B
6.5 Microbicide test
According to the relevant methods in GB15979 Sanitary Standards for Disposable Sanitary Products or Disinfection Technical Specifications
7. Testing regulations
7.1 Before shipping from the factory, the technical inspection department of the factory should carry out the inspection of key items in accordance with the standards. After being confirmed qualified, the factory quality inspection department can issue a certificate,them it can be inspected in the warehouse or shipped from the factory.
7.2 Take one-off production to produce a complete package of products as a batch, and each batch of products is randomly sampled at 3 times the inspection amount for random inspection (except items 5.2, 5.3.2, and 5.5). If one of the results fails, you must double the sampling and repeat Inspection, if the result is still unqualified, the batch of products is judged to be unqualified
7.3 Product inspection is divided into factory inspection and type inspection. The factory inspection is the inspection except 5.2, 5.3.2, and 5.5; the type inspection includes all the inspections required by this standard. One of the following conditions should be tested
When a new product is trial-produced and put into mass production, after formal production, if there are major changes in raw materials and processes that may affect product performance and quality, when the national quality supervision agency and statutory supervision and inspection agency request type inspection
7.4 If the supplier and the buyer have objections to the product and cannot reach an agreement, they can negotiate an arbitration inspection, and the inspection result is final.
8 . Marking, package, transportation and storage
8.1 Obvious signs should be on the package, including: product name, factory name, factory site, standard number, batch number, production date, storage period, product instruction manual, etc.,All should comply with the relevant provisions of GB191.
8.2 The volume capacity are 500m1, and special volume can be customized according to customer requirements; the direct packaging is plastic bottles, outer packaging is cartons, with lists, product certifications, product introductions inside, etc.
8.3 Transportation
No squeeze, seal, store in a cool, ventilated place
8.4 Storage
Keep away from light, sealed, moisture-proof, store in a cool, dry place. Product effective storage period is two years
9 . labels and instructions
It should meet the requirements of the Ministry of Health's Regulations on the Management of Labeling Instructions for Disinfection Products (05 editions)
- Size: 0.049
- Place of Origin: Heze City ,Shandong Province
- Brand Name: Huangying
- Model Number: 500ml
- Weight: 23.000 kg
Name: | Hand Sanitizer Gel |
Net Weight(bottle): | 500ml |
Unit Price(bottle): | RMB11.2/bottle |
Specification(box): | 40bottle/box |
Net Weight (box): | 20KG |
Gross Weight (box): | 23KG |
Volume(box): | 0.55m×0.425m×0.21m 0.049m³ |
Daily output: | 60000 bottles |
Purchase 500 thousand delivery time: | 40,000 bottles per day, 500,000 bottles in 12 days |
Label: | Huang Ying |
Application Field: | Hospitals, pharmacies, clinics, supermarkets, schools, enterprises, etc |